“
Fabiana Toledo
It was the first work experience using the GO!FIVE® tool. I considered it excellent mainly in the aspect of agility and data integrity. Great tool, mainly when teams are allocated to physically separate plants. Hypera Pharma
“
João Paulo
We have FIVE as a great partner and consultant. I strongly say that FIVE professionals brought a new culture of information security and data integrity to Fagron. Thank you very much. Fagron
“
Graziela Amaral
There is a great ease in doing business and work with FIVE, due to the high level of understanding and training of collaborators. Ceva
“
Thais Ushikusa
Seriousness and competence. Bayer
“
Janine Machado
Excellent job! Galderma
“
Gabriela Alencar
The work was carried out in an agile and assertive manner. We always had the support of FIVE during the project. Galderma
“
Abel Henrique Silva
A company committed and always meeting all the deadlines proposed for the project. Gemini
“
Luciana Pereira
Very satisfactory, with compliance with the deadlines and specialized team. Gemini
“
Jair Calixto
FIVE is a serious company and committed to the quality of medicines through the systems validation services provided to the pharmaceutical industries. Refined expertise and a high degree of knowledge in validation. IBBPF
“
Carina Brolazo
We had all meetings attended and proposals delivered on time. Galderma
“
Lucas Reinaldi
Very didactic and attentive tutor. Congratulations. Forlogic
“
Thayana Gimenez
We had positive feedback from many people who attended the webinar. The subject was specific, according to our reality. They dominate the subject a lot! Very good! Forlogic
“
Mila Palamidovska
The GO!FIVE® platform is easy to use, documents are easily accessed. Alkaloid AD
“
Darko Atanasoski
The remote validation with FIVE was quite a rewarding experience all together. Alkaloid AD
“
Nikola Dimovski
The partnership proved to be a complete success. Alkaloid AD
“
Graziela Amaral
There is a great ease of doing business and working with FIVE, due to the high level of understanding and training of their employees. Ceva
“
Ricardo Margonato
The FIVE team, besides their great knowledge, is always very committed to the tasks, facilitating the progress of the project. Globe Química
“
Tatiana Cardozo
I am very grateful to the "FIVE" team for their excellent leadership with the project. FIVE's success is due to the commitment and efficiency of its work. Thank you very much for your help in our many questions and defining courses for actions. BIOMM
“
Letícia Trova
The FIVE team gave all the necessary support so that the validation happened as planned and within the agreed deadlines. It was an excellent team work. Catalent
“
Fernanda Ferreira
Once again I would like to thank FIVE for their understanding and professionalism, especially our project colleagues who were extremely assertive and dedicated, contributing a lot to the success of this project! Torrent
“
Kelly Ido
Working with FIVE on this project was an amazing experience! Ipanema
“
Adriana Costa
I thank the entire FIVE team for their attention and commitment. The analysts and supervisors are wonderful professionals. Fundação Bahiafarma
“
Everton Donel
I would like to congratulate the FIVE team for being extremely professional. Pfizer
“
Augusto Mendonça
FIVE's team is to be congratulated for the guidance and support with our computerized system validation project, the use of the GO!FIVE® platform also helped a lot in the success of this project. Laboratório Wesp
“
Ricardo Margonato
This project ran effortlessly and the FIVE team was always available to help. This type of project was, by its idiosyncracies and complexity, a real drain of time, so a realistic, well-managed and well-trained team, along with the programming are just some of the reasons for our success. The GO!FIVE® platform helped to give speed and confidence. Globe Química
“
Rodrigo Endo
Efficient and assertive, excellent! The portal of GO!FIVE® is very good, congratulations! Great validation analysts and we must congratulate the management. Toledo / P&G
“
Lais Yamamoto
We had an urgent project with a short deadline and the FIVE team met our needs and delivered all the requirements on time with the desired quality. Thanks for your partnership. Thank you very much! Galderma
“
Fernanda Ferreira
The realization of the project by the FIVE team was of extreme dedication and cooperation. On behalf of Torrent, I would like to thank all the specialists for their professionalism, and being efficient! The GO!FIVE® system is intuitive and was of great value. We are very happy to have finished this journey with FIVE, and hope that soon we will be together for other successful projects in the future. Torrent
“
Leni Carvalho
Congratulations, very helpful staff. BIOMM
“
Rubens Fernandes
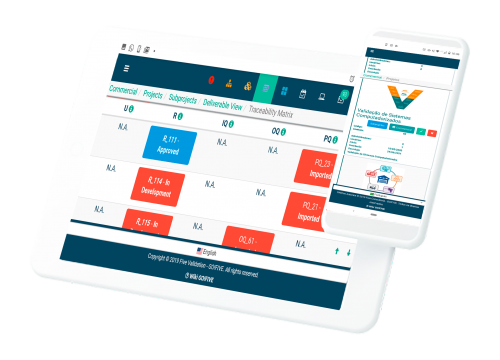
One of the most important challenges in any computer system validation project is the control and management of the documents captured in the project, as well as the generation of documents and their mandatory approvals. In the RBBL project, with the use of the GO!FIVE® platform from FIVE Validation, this challenge is no longer a problem. The platform properly managed all the documents and their versions, as well as all the required approvals at each stage of the project. We used the GO!FIVE® platform during 9 months of our project, without the occurrence of any systemic bug. EMS
“
Ingrid Oliveira
It was a very valuable training for me, especially in the automation part, which I didn't know much about. Fresenius Kabi
“
Lucas Rodrigues
Excellent training, very didactic and interactive. Being conducted onsite facilitated the interaction and learning a lot. Fresenius Kabi
“
Tatiana Cardozo
The FIVE employees' expertise, who were involved in the project, especially the system to be validated and the validation concepts, helped BIOMM to carry out the project effortlessly. BIOMM
“
Ellen Costa
It was a challenging and very difficult project for the company, Five's support and critical vision were essential for this project's success. HalexIstar
“
Graziela Amaral
The entire project was well-organized in all its facets, meeting all of Ceva's needs. The conducting of the validation activities occurred pro-actively, objectively and quickly, both in the aspects of interpersonal relationships and the technical discussions to resolve problems encountered during the project. The whole process of documentation was logical. Review and execution with GO!FIVE® was very easy to do. Ceva
“
Isabella Azevedo
This project was my first experience with FIVE, and I have nothing but praise for them. I was given the project in the middle of the process and the people at FIVE gave me all the support that I needed to understand the stage where our group was at, and were very patient in showing me how to execute the tasks in the application. I thank FIVE for all their commitment and dedication. Thank you so much! Catalent
“
Cristiane Vargas
We were always very well treated during the meetings and during the execution phase of the project. Those responsible demonstrated total mastery of the subject and competence. Congratulations to the team! Espírito Santo Distribuidora
“
Carlos Junior
Very satisfied. Prati Donaduzzi
“
Sibeli Soares
Simply efficient Catalent
“
Vinicius Neugebauer
I think the system is nice and easy to use from the point of view of those who go into it to approve documentations. Takeda
“
Luciana Gonçalves
It was our first experience with external validation and it was great; very easy and well organized. Laboratório Wesp
“
Breno Gine
Very productive contents. Ibramed
“
Juliano Oliveira
Very valuable content. Ibramed
“
Vinicius Neugebauer
The process change of document elaboration, items discussion and tests execution was more efficient, because the strategy was to follow the matrix lines, speeding up the validation process and activities management, while working on improvements in parallel. The work of the validation professionals was exceptional. Takeda
“
Gabriela Sanches
I chose FIVE due to the good reputation in the market and due to previous participation in trainings that were very well conducted. Boehringer Ingelheim
“
Felipe Almeida
I was attended to by a great professional who explained very clearly and patiently the whole process to our team. Pharmalog
“
Diego Araujo
I was very well attended to and had great support. Pharmalog
“
Rafael Moraes
The technical knowledge of the FIVE team and FIVE's exclusive management tools enable the validation of computerized systems to be conducted safely and systematically. Eckert & Ziegler
“
Maurício Barth
First-rate team! The GO!FIVE® platform is a very good tool for System Validation. Prati Donaduzzi
“
Tiago Conceição
Working with GO!FIVE® was a great experience; it significantly streamlined the 'bureaucratic' documentation processes, and its traceability features were very beneficial. Prati Donaduzzi
“
Cassiana Mazzer
I would like to thank the FIVE team very much for all the care they had in this project. You were truly exceptional in meeting deadlines, attention, patience, planning, among other aspects. Solabia
“
Lais Yamamoto
Thanks for your partnership. Galderma
“
Rodrigo Endo
Great job. Toledo / P&G
“
Gerusa da Silva Lopes
I emphasize the dedication of the professionals involved in the project, including their professionalism and willingness to answer questions and propose solutions. Porto Seco Centro Oeste
“
Rafaella Nascimento
In general, this project was very good, FIVE's team provided all necessary assistance during the project. They clarified all questions and provided several alternatives during the discussion, helping us to solve our problems. Cargill
“
Cristiane Vargas
We were always very well-handled by FIVE consultants. They were very helpful in assisting us with our project and questions. Espírito Santo Distribuidora
“
Priscila Gouveia
An easily accessible system for both approving evidence and navigating O Boticario
“
Gabriela Theis
Really interesting software! FGM Dentalgroup
“
Evangelos Mantadakis
Great presentation! Great overview! Cannavigia
“
Polyana Marçal
The use of GO!FIVE®, as well as the libraries provided in the system, has resulted in a significant improvement in the efficiency of generating subject-specific documents and simplification of review and approval routines. Our expectation is to manage all validations in the software, as well as maintenance and periodic reviews in the future Hypofarma
“
Camila Joaquim
Great commitment from the team in delivering the project, along with excellent interpersonal relationships Prati Donaduzzi
“
Vanessa Scaldelai
A team that assisted us was very helpful Stryker
“
Thalyta Almeida
The whole team is very friendly and helpful. They also have great technical expertise Ortofarma
“
Victor Rodrigues
"We carried out a high-impact project in partnership with FIVE, which, through the use of the GO!FIVE® software in the execution of qualification and validation tests, provided us with a 20% reduction in workload allocated to these activities, as well as saving one month of work from various teams while meeting internal quality standards." Organon
“
Estanis Ferrater
"Great professionals, have a broad validation knowledge, produce excellent documents, have initiative and take the right decisions when needed, and help the rest of the team above what should be expected from them. Happy to work with FIVE!" BeiGene
“
Rafael Lima
"Extremely organized work, what was agreed upon was provided." LM Farma