GO!Startup Program
FDA, EMA and WHO Compliance for Startups
A complete package that gives you access to software, training, support, and consulting hours to meet your FDA, EMA, and WHO validation compliance requirements: 6x faster.
Save 75% of time and money while increasing compliance.
*By clicking the APPLY NOW button, there is no financial commitment. The final discount will be sent after evaluating the criteria.

Find out if you’re eligible*:
- Medical Device or HealthTech companies**
- Final product that must be registered with regulatory
- Up to 20 employees including founders, partners, and interns
(*) Eligibility is subject to FIVE Validation approval
(**) Not applicable to Biopharmaceutical companies.
If you do not meet all the criteria, our team will evaluate and give you a proportional discount or find a tailored way to support you. Click here to schedule a meeting.
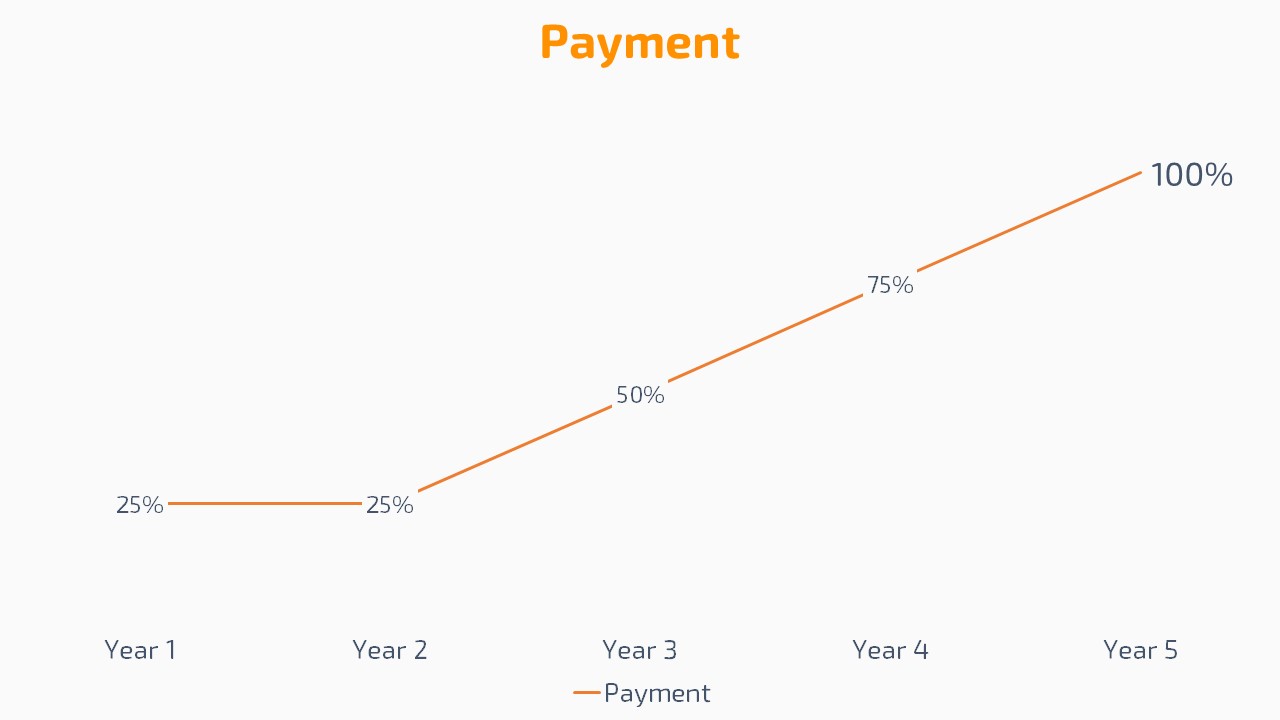
Start paying list price after 4 years
FDA, EMA, and WHO Compliance for Startups
Compliance shouldn't become a burden
Startups and scaleups must meet the same regulatory and validation requirements as big corporates, but with a much smaller team. This distracts the entrepreneurs and prevents them from focusing on their core business.
We understand the startup ecosystem
And that’s the reason why we’ve created GO!Startup Program; to help and empower Medical Devices and HealthTech startups, save up 75% of time and money and increase compliance.
Do it right the first time and accelerate time-to-market
In addition to validation/qualification being a requirement for the registration and commercialization of products, a robust set of documents can get your approval faster and without adjustments.
Let your team focus on its product
Building and maintaining validation documents take time, so use our database with more than 15 years of experience in the field.
To learn more about GO!FIVE®, click here.