Why speed in validation activities is a competitive advantage

Why speed in validation activities is a competitive advantage
A term often used in industries is time-to-market, which is the amount of time from the conception of a product to its release.
It is common knowledge that market pioneers enjoy some advantages in terms of market share, revenue, and economic growth.
Product release timing is everything, considering its innovation, development, and compliance.

Without validation, the biopharmaceutical and medical device industries cannot register or produce their products.
In the traditional way, it is common to take up to 8 months to complete a validation study. With the use of a system such as GO!FIVE®, a cloud validation software, the content inside the database (risks, requirements, tests and others) allows one to carry out validations and qualifications 6x faster.
The importance of time-to-market
In a classic study, McKinsey & Co found that a product which is six months late to go to market loses 33% of its profits in five years.
In a second scenario, if the same product is launched on a scheduled date, but with 50% overbudget, there is only a 4% reduction of profits.
As mentioned before, without validation it is not possible to make biopharmaceuticals available in the market. So streamlining the process can be key to making a difference in your company.
To support clients in projecting and evaluating investments in the validation/qualification and business decision-making areas, FIVE team has prepared an interesting article on the return on investment (ROI) for paperless validation in a single platform, click here to view.
Quality as an ally for value creation

Over the years and with technological advances, quality has been pressured to increase its efficiency, adopt digital technologies to reduce directly identifiable costs.
As a result, paperless workflows, data automation and several other technologies were adopted to achieve these aims of reduction.
Despite being more positive than in the past, this mentality of viewing only visible costs has prevented taking full advantage of real opportunities.
One perspective is that regulatory requirements which are met contribute to the success of the business. Currently, processes are still built reactionary which slows down the business and increases costs.
In another study released by McKinsey & Company, an intelligent quality system can have a tangible impact on profits and accelerate time-to-market by more than 30% and increase the supply chain production capacity by 20-30%.
Smart quality combines advanced technologies, standardization, process, and flexible ways of working, and allows the creation of value for the company at a fraction of the cost years ago.
Life Science companies that plan to increase their portfolio will consequently need to validate these new processes, qualify, and validate the equipment and systems involved.
Having teams work five times faster and being able to take on a larger scope of work with less effort can support this expansion without the need to increase the structure.
By streamlining this formal process while maintaining compliance, resources focus on higher-valued tasks: R&D gains more time to innovate, engineering to develop new processes, and the entire team will have engaged quality, since data is easily accessible, and the requirements are clear and ready-made.
Don't start your validation and qualification projects from scratch.
GO!FIVE® is the only solution with risks, requirements, and tests management available. See more here.
‘Haste makes waste’
As explained in this article, some costs of non-quality are obvious and easily visible. However, there are several hidden costs that weigh more heavily on the company, as well as regulatory and sanction risks, being the submerged part of the iceberg.
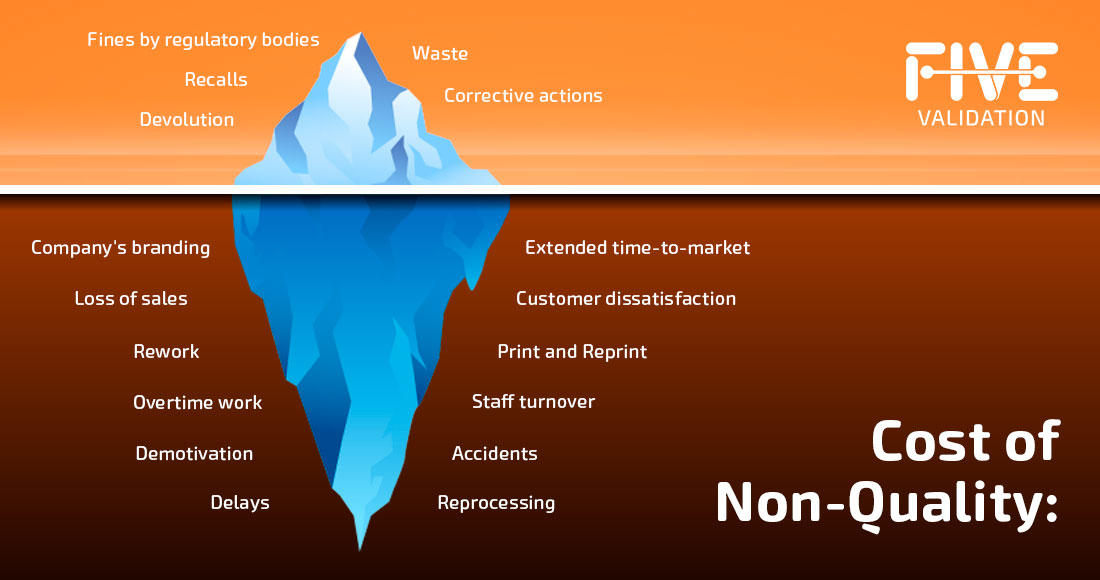
The visible part is already challenging, but the hidden, submerged part also deserves attention, as it consumes human, financial, time, and energy resources and can impact the company's image and value.
There is a popular saying “haste makes waste.” However, speed is not always a bad thing. For example, with GO!FIVE® software it's possible to perform validations at least 6x faster, because it has pre-formatted validations built right into the database of various systems, equipment and utilities taking into consideration good industry practices.
There is no magic formula! The system was developed with more than fourteen years of experience compiled into a fast, safe, and easy-to-use platform.
Productivity is the key
Automation and digitalization of manual activities have been presented as a useful solution for productivity gains. It is important to note that the use of technology does not mean the end of work for people, but the prioritization of their time in important actions.
Higher productivity, lower cost, and time optimization: In GO!FIVE®, the validation professional has a knowledge base, electronic tests, dashboard and automatic document generation.
Integrate areas and promote team collaboration! Click here to learn more.
If you've never tried digital validation software before, now is the time.
Schedule a meeting with us and learn more about the software that streamlines compliance and empowers teams all around the world. Request a demo.
Reference:
MCKINSEY&COMPANY. Smart quality: Reimagining the way quality works. January 25, 2021. Available at: <https://www.mckinsey.com/industries/life-sciences/our-insights/smart-quality-reimagining-the-way-quality-works>. Accessed on 26 april 2023.
TCGEN. Time To Market (TTM): What it is & Why It’s Important. [2023]. Available at: <https://www.tcgen.com/time-to-market/>. Accessed on 26 april 2023.



