Get Your Data Integrity Basics Down for Success

This article was written by Silvia Martins and disclosed by PDA (Parenteral Drug Association) https://www.pda.org/pda-letter-portal/archives/full-article/get-your-data-integrity-basics-down-for-success.
In recent years, regulatory investigators have observed an increase in violations involving data integrity (1). Inconsistencies related to data integrity pose risks to product safety, effectiveness and quality. At the same time, pharmaceutical manufacturing is growing ever more digitized, raising the criticality of data integrity even further. In light of these technological changes, it pays for companies to invest in a data integrity strategy.
The major regulatory agencies continue to focus audits on data integrity and remain committed to monitoring fraud cases around the world, even sharing cases of nonconformity with other agencies to get a global picture of how the issue has been addressed by industries.
Naturally, pharmaceutical companies are taking a closer look at the state of data integrity within their companies. The first task that must be considered when developing best practices for data integrity is to record the current situation of the company via data inventory. This entails reviewing compliance based on current procedures for managing critical data.
Next, the validation and QA teams must work together jointly to author a risk analysis to define which data falls under GMP and GDP (GxP) and are part of batch release. This step is intended to prevent all company data, even those not subject to GxP, from being included the data integrity process, as this would increase the cost and complexity of the production process. The master plan and procedures should consider this information so that the quality system has clearly defined which data will be subject to data integrity best practices.
The data supporting such decisions need to be complete as well as accurate, legible, contemporary, original and attributable, i.e., follow ALCOA principles. These basic GxP principles ensure the reliability of the data and they are not new.
It is essential to invest in employee training
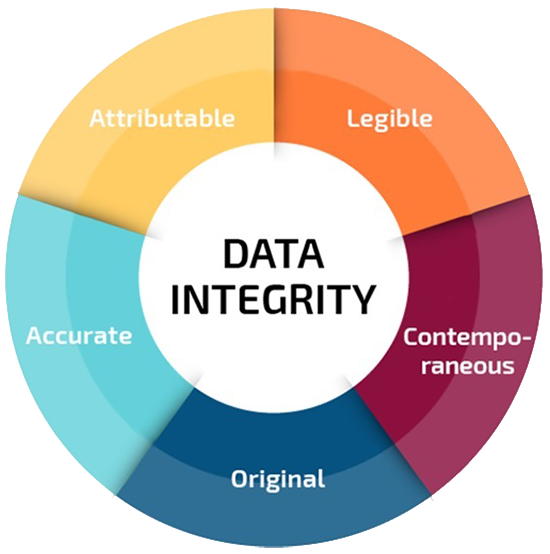
ALCOA Principles of Data Integrity
The ALCOA concept is based on the accurate, complete and consistent recording and management of a data or information, either on paper or electronically.
The data on which these decisions are based should therefore be complete as well as being attributable, legible, contemporaneous, original and accurate, commonly referred to as “ALCOA.” The basic principles and the related good practice expectations that assure data reliability. Below are examples of each of the ALCOA principles:
Attributable – Data can be assigned to the individual performing the task
Legible – Data can be read by eye or electronically and retained in a permanent format
Contemporaneous – Data is created at the time the activity is performed
Original – Data is in the same format as it was initially generated, or as a "verified copy," which retains content and meaning
Accurate – Data is true/reflective of the activity or measurement performed Some actions may be taken within the production process that contribute to data integrity requirements. These can consist of the following:
• Keep a watch available in activity areas to avoid mistaken notes of time
• Facilitate operators' access to documents related to the activities they perform to avoid recording data after the fact
• Control unfilled forms to minimize the risk of wrong information recorded
• Control access to computer systems to prioritize traceability of data
• Avoid transcription of data and using printers directly connected to equipment
• Facilitate access to raw data by those responsible for data review to minimize regulators finding nonconformities
8 Essential Steps for Data Integrity
So, how can pharmaceutical manufacturers develop a culture that supports high levels of data integrity? There are eight steps that must be taken to build such an environment.
1. Ensure limited and authorized individual access control
2. Define, clearly, complete records
3. Define, clearly, access profiles
4. Evaluate and record the evaluation of all relevant GxP records
5. Implement, monitor and review critical audit trail logs before product release
6. Backup data regularly
7. Include information security features and integrity in computer system validation approach
8. Train users
Regarding the last point, it is essential to invest in employee training to deal with relevant data integrity requirements and to create an in-company culture of attention to procedures so that employees feel responsible for the integrity of the data generated in their area, production stage or analysis.
Go Digital to Ensure Data Integrity

Well-implemented computer systems can streamline processes and make data entry more efficient and organized. Tools in compliance with FDA 21 CFR Part 11 and robust data integrity policies can help improve processes, no matter what stage of digital transformation journey a company is in.
A robust data integrity initiative can bring a company closer to digital transformation since companies are automating their production processes with incredible speed to optimize time and reduce costs. Paper-based processes remain slow and susceptible to human error. Companies that automate their processes and embrace digital transformation will see gains in quality while companies still reliant on manual processes will experience low efficiency due to delays from outdated manufacturing processes and increased regulatory risk. Pharmaceutical manufacturers must implement innovative software and systems that streamline paper-based activities to increase efficiency and maintain compliance.
While other manufacturing industries discuss Industry 4.0 and big data, pharmaceutical manufacturers are still securing the integrity of their regulatory records. Data integrity compliance tends to be reactive and many see data as a liability. Yet data integrity can be a great opportunity for digital transformation.
For years, pharmaceutical manufactures have claimed they had difficulty in adopting innovative manufacturing solutions due to regulatory requirements. Now, global regulators are increasingly adopting risk-based approaches that offer companies tools such as risk assessments and control strategies for managing product quality. Efficient analysis of stored data enables continuous improvements.
Data integrity and digital transformation go hand-in-hand. For example, data integrity reinforces GMP in automation and IT. By incorporating quality culture principles, manufacturers can ensure that the entire organization is in line with current challenges and future improvements.
Data Transformation for Compliance
Many businesses today are scrutinizing their operations to figure out how to join the digital transformation revolution. They understand that to become more competitive, they need processes that are integrated and scalable. They understand that controlling and making use of robust data is the key to success.
Unfortunately, poor data practices, which can cost a lot of money, are often overlooked. When it comes to the impact caused by poor data quality, the figures speak for themselves.
To turn that enormous loss into opportunities, CIOs and CEOs need to better operationalize data at enterprise scale— making qualified, clean and reliable data available to employees to then analyze to make fast, informed decisions. With the new emphasis on agility through digital transformation, CEOs now have the power to enable rapid change within their businesses by developing digital strategies with data at the core. These leaders have to change the departmental view that data is solely an asset used primarily by data scientists and expand it to cover data used across the entire enterprise.
CIOs and quality managers need to rethink their role within the broader organization—shifting from simply being a caretaker of utility-type technologies that run the business to be facilitators that help users leverage data to gain performance and make good decisions.
References
1. Data Integrity: SINDUSFARMA Guideline for Pharmaceutical Industry (Brazil, Portuguese language)
2. European Medicines Agency Questions and answers: Good manufacturing practice – Data Integrity
3. WHO Annex 5 Guidance on good data and record management practices.



