
Validate from anywhere, in compliance with FDA and EMA

Remote work, currently and from now on, will no longer be considered an option for professionals and companies.
The companies that were gradually inserting this modality of work in their routine are accelerating this process, with tools that enable the integration of the team and productivity, without the need for the physical presence of the professional to perform the work.
And when well implemented, with proper training in the selected tools, remote work provides greater quality of life for the team, has the ability to retain highly qualified professionals, provides specialists from anywhere in the world and reduces the number of people in the environment.

This new behavior imposed on us by COVID-19 has a direct connection to digital transformation, which was already happening in an accelerated way for some sectors of the industry and now becomes the central agenda for businesses that were still crawling in this direction. The challenges in this scenario are as great as the opportunities and it is up to the professional to prepare to remain productive, even without being present, and it is up to the companies to provide tools that meet this need and transform this challenge into an opportunity also for the business. One good turn deserves another!.
For some activities, remote work remains unfeasible and in these cases adaptations are required in the routine and in the work environment, but for validations of management systems, software and, equipment connected to internet, it is already possible to perform these activities completely remotely.
Remote Validation versus On Site Validation
Since the beginning, validation and related activities were performed in the classic way, based on paper and professional's indispensable physical presence. Over time, tools emerged to digitize part of these activities, but still required the professional to be present at the site, to carry out these works.
From the point of view of the current situation, this initial evolution of the form of validation still means expenses with displacement of the professional and risks of contamination, with the handling of documents by different people. On the other hand, it is already possible to carry out different types of validations in a totally remote way, increasing security for professionals while increasing the dynamism of work.


By carrying out completely remote validation, you eliminate the risk of contamination among professionals, as there is no circulation of documents between different sectors and people.
One of the phases of classical validation that considerably increases these risks is the execution and approval of tests.
At this moment, the paper document has to pass through different hands to be evaluated, whereas in the totally remote validation this phase of the project can be carried out completely online, in accordance with the rules of FDA and EMA.
.
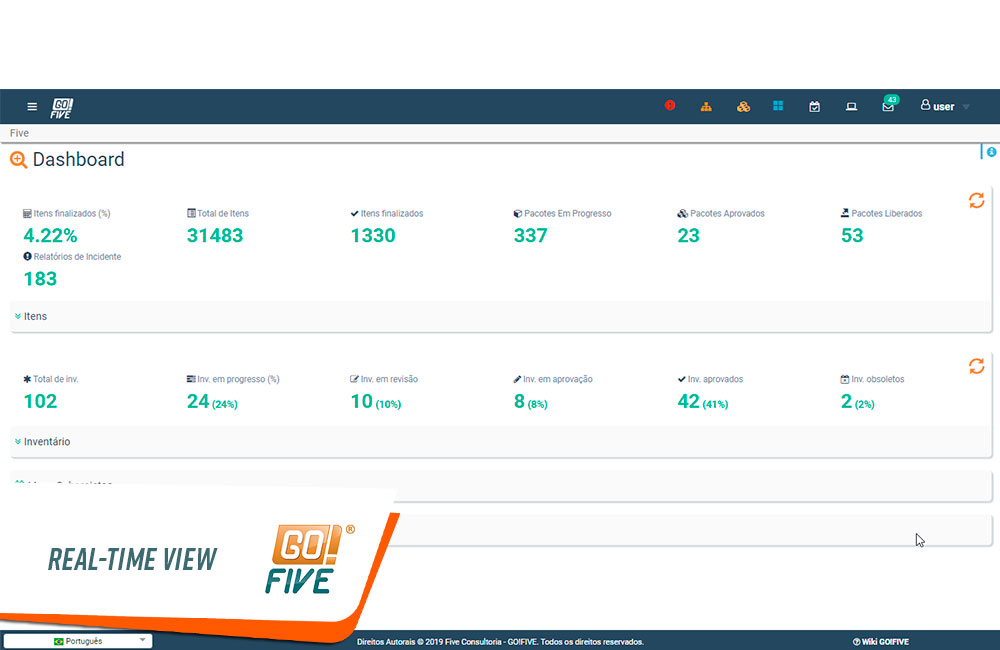
Another advantage of remote validation is the possibility to monitor the progress of these activities in real time. You can create and run the tests, send them for review and approval by the person in charge and collect signatures in a process that takes place entirely digital. A feature that becomes even more interesting when we talk about global teams, working on different sites.
Data integrity compliance is also an important achievement when using a paperless validation software.
Other benefits of fully remote validation
- increase in the number of validations, without increasing the team;
- validations performed 5 times faster;
- reduction in the cost of projects;
- cloud platform, access to the projects from anywhere in the world;
- features that facilitate maintaining the validated status;
- risk-based approach, in accordance with GAMP5® guide;
- paperless validation software that is compliant with FDA and EMA

We live in a time when innovation is the way to carry out validation activities, while ensuring the safety, health of professionals and the progress of the company's projects. In this scenario, totally remote validation appears to meet these requirements that supports inserting Life Science industries in the era of digital transformation.
Do you have a validation project?
Learn more about how Remote Validation can be applied.
Get in touch through the chat on the side.
Get in touch and learn more about remote validation.GAMP5® is a guide that has its intellectual rights reserved by ISPE™. Available for purchase at ispe.org

