Track and Trace for Pharmaceuticals in Brazil

In general, the serialization and traceability projects consist of obtaining information from each commercial drug unit in its entire chain (manufacture/import, storage, distribution and dispensation).
In this way, it is possible to check the regulation of the drug, that is, if it is a registered product that was produced or imported by an authorized company and all its movement in the logistic chain until it reaches the consumer or health unit.
In this way, it is possible to check the regulation of the drug, that is, if it is a registered product that was produced or imported by an authorized company and all its movement in the logistic chain until it reaches the consumer or health unit.
It is important to highlight that the SNCM (National Drug Control System) does not address the fiscal movement of drugs, focusing on the physical movement process, so it does not need to be communicated when there has been no physical movement or change/handling of cargo that can modify its sanitary conditions..
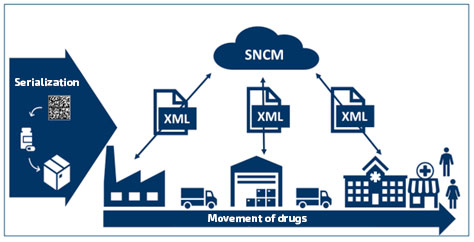
Figure 1: Serialized drug movement chain integrated into the SNCM (adapt. ANVISA, 2020)
Which drugs will be subject to SNCM control?

As required by law, the competent federal health surveillance agency will determine the categories of drugs that will be subject to control.
On August 23rd, 2021, ANVISA consolidated the Normative Instruction - IN No. 100, which establishes the drugs subject to the National Drug Control System (SNCM) and the deadlines for serialization and for starting the communication of records of instances of events.

On August 23rd, 2021, ANVISA consolidated the Normative Instruction - IN No. 100, which establishes the drugs subject to the National Drug Control System (SNCM) and the deadlines for serialization and for starting the communication of records of instances of events.

Phases and deadlines of the serialization and traceability project
All regularized drugs, with the exception of the one highlighted in Art 4, must be mandatorily serialized for the purpose of reporting the instance record of events in the movement chain to the National Drug Control System (SNCM) by April 28, 2022. Therefore, there seems to be no longer the concept of a module “implementation SNCM” and “definitive SNCM”, and it is not clear how the L5 tests with the ANVISA server will be. Perhaps the first tests can be carried out with a real product.
The deadlines are challenging and according to article 4, until April 28, 2022, 100% of the batches must carry out the appropriate communication of events in the SNCM system. Validation, in addition to being a regulatory requirement, can support proof of these percentages to the regulatory body.
The fact that the serialization plan must be made available within 30 days after the platform is made is highlighted, that is, the sooner companies prepare, the easier the availability and planning will be. Do not leave the planning and quotation of activities to the last minute.

Implementation of the serialization and traceability project
The implementation of traceability takes place through the serialization and aggregation of products. Depending on the automation of the line and its complexity, the project can be ‘more or less’ challenging.
The traceability project is composed of several levels, which is the hardware and software architecture necessary for this relevant information of drugs, are intact, complete and traceable. Try to identify which layer(s) will be needed and which equipment, devices, packaging lines and software will be involved.
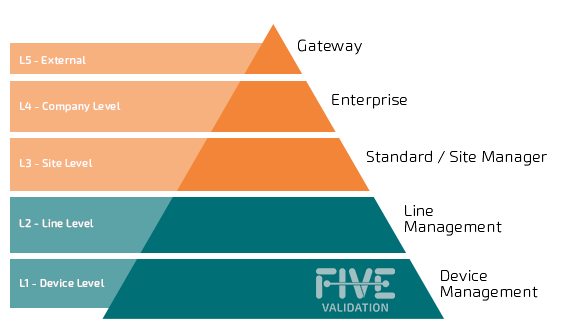
Validation of computerized systems
According to Guideline n° 01/2020 of October 7, 2020 (Guideline of the National Drug Control System), they must submit the client system1 to computerized systems validation:
1According to Guideline n° 01/2020 of October 7, 2020 (Guideline of the National Drug Control System), the client system is characterized by the technological environment that must be operated by the member of the drug movement chain or respective attorney, with the purpose to perform this communication.
2According to Law n° 13,410, Art. 3: the establishment that fails to communicate any information regarding the movement of drugs commits a sanitary infraction.
- Registration holder;
- Distributor;
- Dispenser.
1According to Guideline n° 01/2020 of October 7, 2020 (Guideline of the National Drug Control System), the client system is characterized by the technological environment that must be operated by the member of the drug movement chain or respective attorney, with the purpose to perform this communication.
2According to Law n° 13,410, Art. 3: the establishment that fails to communicate any information regarding the movement of drugs commits a sanitary infraction.

Use of paperless validation in the serialization and traceability projects

The serialization and traceability project involves a lot of technology; however, many companies still choose to carry it out in the traditional way, on paper, which makes the process time-consuming and bureaucratic.
This project has different characteristics, because it has several stages and partial validation releases for the use of the traceability system, since there are communications with different links in the supply chain and respective validations that can occur at different times in the project. The completion of this project will only take place in 2024 when all lines are serialized. Paperless validation is the best solution and strategy. Validation should not be the “bottleneck” in the innovation and technology chain, but rather an ally to ensure compliance at each stage and application.
This project has different characteristics, because it has several stages and partial validation releases for the use of the traceability system, since there are communications with different links in the supply chain and respective validations that can occur at different times in the project. The completion of this project will only take place in 2024 when all lines are serialized. Paperless validation is the best solution and strategy. Validation should not be the “bottleneck” in the innovation and technology chain, but rather an ally to ensure compliance at each stage and application.
Briefly, each line that will be inserted may eventually include new equipment and systems, have other players in the chain, different business rules, new links in the chain and communication with CMOs (Contract Manufacturing Organizations), that is, outsourced companies for the drug manufacturing.
When serializing different batches, we must assess whether there was an impact or not, which will depend, for example, on the type of product, its presentation, whether all or part of the manufacturing is outsourced, among several other factors that may or may not modify the validation already performed.
This behavior is typical of a project that requires releases in phases, and multiple validation reports at different times. GO!FIVE® has a partial release function to facilitate projects that have a single Validation Plan, a single strategy, but distinct release phases.
When serializing different batches, we must assess whether there was an impact or not, which will depend, for example, on the type of product, its presentation, whether all or part of the manufacturing is outsourced, among several other factors that may or may not modify the validation already performed.
This behavior is typical of a project that requires releases in phases, and multiple validation reports at different times. GO!FIVE® has a partial release function to facilitate projects that have a single Validation Plan, a single strategy, but distinct release phases.


Some advantages
O GO!FIVE® is a scalable SaaS platform, where it is possible to validate 3x faster following agile methodology. It has over 13 years of consultancy knowledge within the software with pre-formatted validations built into it. Electronic workflow review and approval allows for remote validations most of the time.- Save time and increase compliance;
- Greater compliance with digital data management
- Greater agility with Parallel Flow and Validation per Item
- Partial releases
- Ease of validated status maintenance;
- Test replication functionality;

Below we highlight the top 10 reasons to consider paperless validation:
- More compliance: decrease regulatory risks to businesses and data integrity.
- Faster time to market: with no validation, biopharma and medical device industries cannot register or produce their products.
- More efficient work: ‘right the first time;’ decrease the time to compliance, make projects agile, and possess ‘a knowledge database’.
- Decrease validation costs: faster work, avoid paperwork, no printers, no physical space to store documents, no documentation scanning.
- Remote work: healthier staff and quality of life, online management, connect teams between several countries.
- Easier validation status maintenance: decrease the time to keep validation status with constant update and periodic inspections.
- Easier audits: immediate availability of data.
- Standard documents: maintaining good documentation practices according to GMP guidelines, GAMP5®, for example.
- Easier management: immediate availability of data (online management).
- Sustainable: no use of paper, no printers, no cartridge disposal.
Do you have a Track and Trace project? Contact us by chat on the side!
We hope that the content expressed in this article has been useful for you!IMPORTANT: the validation cycle must be concurrent with the project implementation. “Don't leave it to the last minute”.
Get in touch with one of our specialists via e-mail: [email protected]
GAMP5® is a guide that has its intellectual rights reserved by ISPE™. Available for purchase at ispe.org



