Attain SAP® Validation Easily

Attain SAP® Validation Easily...
Many companies have been struggling to plan their migration journey from SAP ECC® to S/4 HANA® in the last few years. Although the SAP® support deadline has been extended, one of the concerns is the shortage of professionals in the market who have knowledge of the project.
For large companies in the Life Science market, the challenge becomes even greater. Planning conversions of this type require patience, rigorous planning, and responsibility, as it can directly affect good manufacturing and distribution practices
To put the GMP® validation project into context, there are two interesting articles on the subject in our blog:
• SAP ECC® and SAP HANA® – Technical Upgrade
• SAP HANA® ERP Validation®
Basically, we can divide the Computer Validation project into two types, validation of SAP S/4 HANA® (change of database and application) and validation of the migration from Oracle ECC® database to S/4 HANA®.
When companies decide to change both the application and the database, there are two main strategies: the Greenfield approach (implementation from scratch) and the Brownfield (software upgrade that preserves all data and settings).
For the Greenfield, one must remember to retain the data in the old ERP, which may be unfeasible for medical device manufacturers, e.g., implant companies may need to keep batch data for the lifetime of the patient.
For both strategies, it is important to evaluate the advantages and disadvantages, as well as the risks associated. Involving a company that specializes in defining regulatory and compliance requirements can help in the decision-making process, compliance with requirements of GxP Pharma, cGMP guidelines, FDA 21 CFR Part 11, GAMP 5, GxP system validation, and GMP validation guidelines.

GxP validation when done on paper is a laborious, bureaucratic, and error-prone activity that can paralyze an operation.
Although each company has its own unique needs, there are several standard processes within a type of system. For example, a purchasing process within an ERP is usually the same process for many companies.
FIVE has experience and has been supporting clients worldwide in such projects since 2008, due to its vast knowledge in computer system validation and relevant GxP processes in ERPs. Electronic validation projects have been used in various company sizes more efficiently (up to 6x faster).
Besides the challenges mentioned above, the SAP S/4 HANA® migration project usually has a high associated cost. There is a shortage of professionals with validation expertise for this type of project. However, FIVE has several services and delivery methods (flexible scope) to meet the client's demand and needs (cost-effectiveness).

Benefit From Our Experience
The entire project was performed remotely, using the GO!FIVE® system (Validation Lifecycle Management Software – VLMS, that is cloud-based, fully paperless, with features that increase compliance).
Below are some of the testimonies from this project:
“The GO!FIVE® platform is easy to use, documents are easily accessed" Mila, IT
“The remote validation with FIVE was quite a rewarding experience all together” Darko, QA Validation
“The partnership proved to be a complete success" Nikola, IT Director
Through the experience of several performed projects, FIVE has created a knowledge database that is constantly updated. To learn more about paperless validation software click here.

Learn More About the Supply Options for This Type of Project
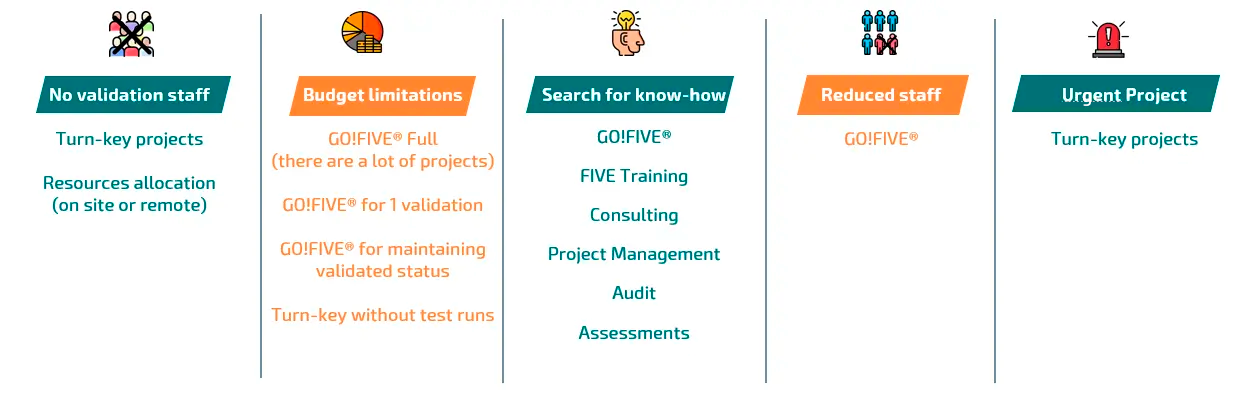
See How Our Services Facilitate the Client Experience
Defined Scope Validation Project (with or without test runs)
Create and build documents through narrow-scoped package and issue all validation deliverables or specific ones. This is useful when one desires to make the service more flexible and optimize costs in validation. FIVE can work with you to discuss risk scenarios and propose mitigations for transactions and processes. For example, your team could perform testing protocols and transfer know-how from us during the project.
GO!FIVE® Subproject (1 validation)
Import specific libraries for the system that you want to validate. There is a specific library for SAP™ ERP. This means that within GO!FIVE®, there is a ready-to-use SAP™ validation for your company with typical regulated company processes, including typical industry requirements, risks and tests. This may be: purchasing raw material only from an approved supplier; quarantining production materials without permission to use until the QC lab release; master data entries; managing materials through status and warehouses; releasing the finished product for sale and shipping rules.
All workflow is in a single platform, and any document can be extracted at any time. Library is built with the experience of more than 1000 projects executed for more than 200 clients.
• Access to the GO!FIVE® support Wiki portal in the PDF manual, also available in HTML and video formats
• Library of compliance and specific items for the modules/processes that you would like to validate, including data integrity items.
• Support for using the features of GO!FIVE® (24/7).
GO!FIVE® for maintaining the validated status
FIVE can either assist in the project, or grant access to GO!FIVE®’s client to maintain the CSV status themselves.
Even validation projects created on paper can be easily maintained with an e-validation solution. Because it is not necessary to update all items every time there is a new version or change.
GO!FIVE®Full (unlimited number of validations)
GO!FIVE® is a customized digital validation at scale and fully flexible through a purpose-built solution. All workflow is in a single e-validation software and all documents can be extracted at any time. With clicks, one can import pre-prepared validations, according to the process and system to be validated. The library is built with the experience of more than 1000 projects executed for more than 160 clients.
All this is to stay ahead of innovation with the compliance and agility that you need.
Facilitate collaboration across your global teams through a 100% paperless platform with an easy online management of validation activities.
We support and engage you throughout your entire validation journey whenever and wherever you need us. FIVE developed the only VLMS (Validation Lifecycle Management System) that includes a knowledge database and various consulting packages.
• Access to the GO!FIVE® support Wiki portal in the PDF manual, also available in HTML and video formats;
• Full access to libraries;
• Support for the use of GO!FIVE® functions (24/7);
• Access to all courses on our training platform;
• Consulting package is already included.
Click here to learn more about the GO!FIVE® paperless validation system.
Allocation
A client hires FIVE and will be able to define what services will be required according to their needs and audit schedules. In this contracting model, as in all other services, there is a follow-up by a FIVE supervisor who reviews documents and participates in the main discussions and definitions of validation and/or qualification strategies together with the client.
Consulting
We train and orientate new or existing teams in the activities of planning and performing validations, with an option of providing document templates.
FIVE Training
We provide video-based corporate education solutions and online exclusive attention that contribute to mitigate compliance risks, proof to regulatory agencies of the capability of an internal team to conduct validations. We also support the strategic development and performance improvement of Life Science companies.

Please contact us
You must have already noticed that there are several supply options and that the shape and model will depend on the needs and specific issues of each client. So, should you wish to request a quote or clarify any doubts about these options, please contact us via e-mail [email protected]
SAP ECC® and S/4 HANA® software are registered trademarks of SAP in Germany and other countries. All rights reserved.
For more details, click here.



