Verification & Validation – V&V

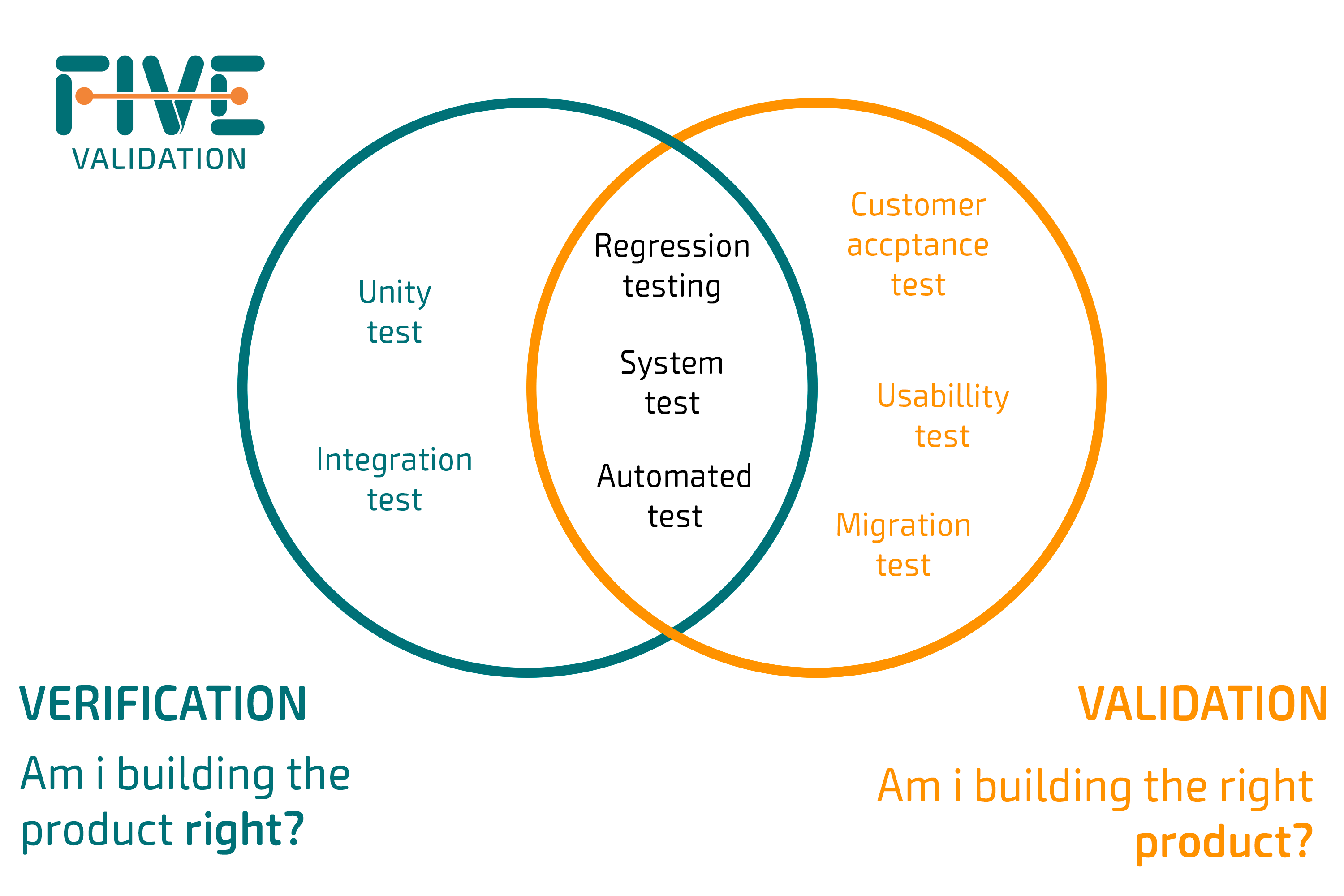
Verification is a process that determines the quality of a product. This step includes all the activities associated with high-quality production, (i.e., testing, inspection, design analysis, specification analysis, etc.). One of the advantages of verification is that it reduces the number of defects that may be found in later stages of development.
Validation is a process in which the user and regulatory requirements are met by the functionality of the software or device. Validation is done at the end of the development process and occurs after the verification has been completed. Validation helps build the right product according to the client's requirements, which in turn, will satisfy their business needs.
Verification and validation (also abbreviated as V&V) are distinct procedures that are used together to verify that a product, service, or system meets all requirements, specifications, and intended purpose (e.g., involving risk management to ensure consumer safety).
To further illustrate this, we can think of the following question for Validation: "Are you building the right product?"; and for Verification: "Are you building the product correctly?"
"Building the right product" refers to meeting user requirements. While "building correctly," is related to the specifications implemented correctly by the object of study.
In some cases, the company does not have a dedicated area for these activities, so Verification and Validation can be performed independently, being carried out by a third party. In this case "Independent Verification and Validation" can be abbreviated as "IV&V".
To determine compliance, it is necessary to have written requirements for both, as well as formal procedures or protocols. It is perfectly possible for a product to pass the Verification phase but to fail the Validation. This can happen when a product is built according to specifications, but these specifications do not meet the user's needs.
Product built incorrectly

Product installed incorrectly
Product built and installed correctly
If some defects are not noticed in the verification phase, these can be detected as failures during the validation process, and appropriate corrective actions need to be taken.
According to FDA, any software used for the design, production, packaging, labeling, storage, distribution, installation, and service of all finished products intended for human use must be validated. Regulatory bodies have specific Verification and Validation requirements, for example, ISO 22000:2018, ASME V&V 40, and others.
Typical documents for the V&V process are:
- Validation Plan
- System Requirements Specification
- Network Diagram
- Functional Risk Analysis
- FDA 21 CFR Part 11 Compliance Analysis
- Design Specification
- Functional Specification
- Test Protocols and Scripts (IQ, OQ, PQ)
- Requirements Traceability Matrix (with risks and tests)
- Final Validation Report
GO!FIVE® is a SaaS platform, where it is possible to design and execute V&V projects digitally. It enables Verification and Validation projects 5x faster following agile methodology because it has risk content, requirements, and testing protocols included in the platform.
Below we highlight the top 10 reasons to consider paperless validation:
- More compliance: decrease regulatory risks to businesses and data integrity.
- Faster time to market: with no validation, biopharma and medical device industries cannot register or produce their products.
- More efficient work: ‘right the first time;’ decrease the time to compliance, make projects agile, and possess ‘a knowledge database’.
- Decrease validation costs: faster work, avoid paperwork, no printers, no physical space to store documents, no documentation scanning.
- Remote work: healthier staff and quality of life, online management, connect teams between several countries.
- Easier validation status maintenance: decrease the time to keep validation status with constant update and periodic inspections.
- Easier audits: immediate availability of data.
- Standard documents: maintaining good documentation practices according to GMP guidelines, GAMP5®, for example.
- Easier management: immediate availability of data (online management).
- Sustainable: no use of paper, no printers, no cartridge disposal.
You can also contact one of our experts by e-mail: [email protected]
Reference:



