FIVE Validation Receives SINDUSFARMA 2023 Award.

Sindusfarma, fundado en 1933, es el Sindicato de la Industria de Productos Farmacéuticos del Estado de São Paulo. Su principal objetivo es defender los intereses farmacéuticos en el Estado y en todo Brasil. Todos los años, el sindicato organiza un evento ceremonial para anunciar los ganadores del Premio Sindusfarma de Calidad, que alcanzó su 27ª edición en 2023.
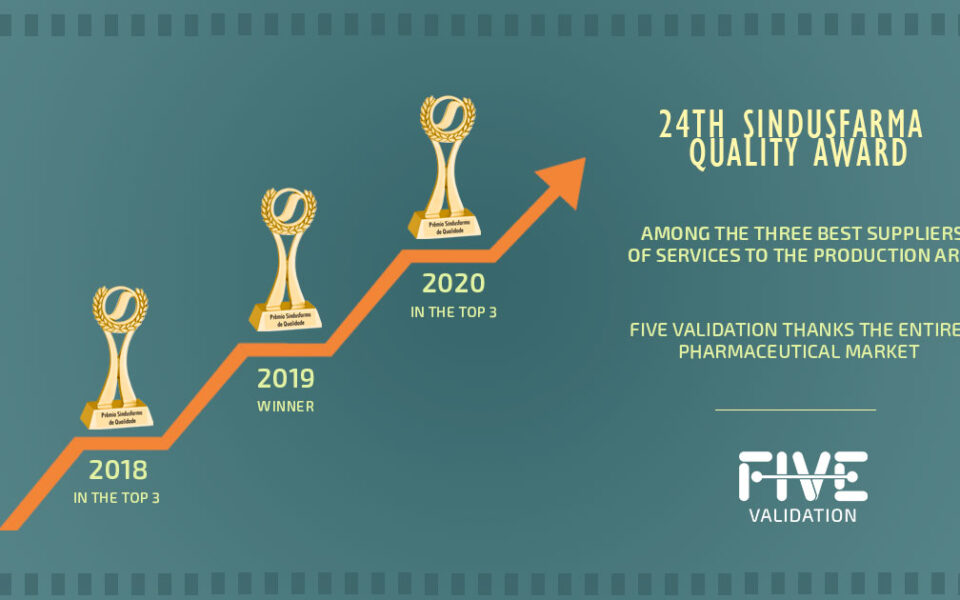
El Premio Sindusfarma tiene como objetivo reconocer a las empresas de todo el país que se destacan en Buenas Prácticas de Fabricación. Desde 2018, FIVE Validation ha solicitado participar en este premio, que ocurre a través de varias fases de selección. En la primera fase, las empresas inscritas son votadas por otras empresas del sector farmacéutico. Las seleccionadas pasan a la segunda fase, en la que se auditan sus respectivos sistemas de calidad.
En 2020, FIVE obtuvo el primer premio en la categoría de "Proveedor de Servicios para la Producción". Incluso antes de la pandemia, la empresa ya operaba en un modelo digital y remoto, incluyendo entregas de estudios de validación a clientes y procesos internos del sistema de gestión de calidad. Este enfoque sin papel ha aportado beneficios independientemente de la situación mundial.

En 2023, Sindusfarma creó la categoría "Software y Soluciones para Equipos de Producción". Como resultado, FIVE ganó el 1er lugar en esta nueva categoría y el segundo lugar en la categoría "Proveedor de Servicios para la Producción". Los diferenciales de este último año de premios incluyen el proceso de implementación de la norma ISO 27001:2022 y la calificación de su infraestructura.
De cara al futuro, el objetivo para el último trimestre de este año (2023) es obtener ambas certificaciones y contratar a una auditoría preparatoria para asegurar que FIVE cumple cada vez más con los principales requisitos de calidad y seguridad de la información.
Bajo la dirección de Sílvia Martins y João Gomes, la empresa avanza en su proceso de internacionalización. El eje EEUU-Europa concentra muchas industrias farmacéuticas, por lo que el avance del proceso de internacionalización permitirá a FIVE atender a un mayor número de clientes que destacan en el sector. De esta forma, FIVE impactará positivamente con su solución de validación en la nube a nuevos clientes de relevancia en el mundo.
Así, el reconocimiento en Brasil a través de Sindusfarma es relevante para el proceso de internacionalización de la empresa, ya que ANVISA es una de las mayores agencias sanitarias y reguladoras del mundo. De esta forma, Sindusfarma realiza su premiación cumpliendo con los requisitos regulatorios de la agencia sanitaria brasileña, lo que a su vez implica que FIVE esté mejor preparada para cumplir con las Buenas Prácticas regulatorias.
Entre los nuevos retos para el futuro de FIVE Validation podemos enumerar: consolidar la implantación de la ISO 27001:2022; aumentar la participación en eventos internacionales de prestigio (uno de los eventos que ya tuvo a FIVE nominada como finalista fue la feria CPhI de Frankfurt en 2022); consolidar el área de Customer Success; y finalizar la calificación de su infraestructura.