FIVE Validation Wins SINDUSFARMA Quality Awards 2020.
FIVE started providing services in 2008 and the first operating procedures were established in 2012. Even without an implementation of a Quality System, FIVE was already studying and addressing the standardization and quality of its deliverables. In 2017, FIVE saw the need to invest more resources in the Quality sector and hired its first exclusively dedicated professional to the department. In this same year, FIVE created more procedures and standard, mapped the processes of all areas/activities as well as job descriptions based on the Brazilian Classification of Occupations (CBO). FIVE developed its Quality Policy for the first time and out of that Effectiveness Evaluations emerged. In addition, FIVE performed Risk Analyses on each document to determine the most critical ones and apply content evaluations to all of them. The documents and activities that were developed were based on ISO 9001:2015. Thus, the Quality System began to take shape and have greater prominence in the company.
To make the quality system more consistent, FIVE conducted an internal audit and initiated a CAPA process suggesting improvements and adjustments, which were subsequently implemented. FIVE also prepared a SWOT analysis highlighting the internal and external, positive, and negative points that affected the company so that it could work to maintain positive points and improve on any negative ones.

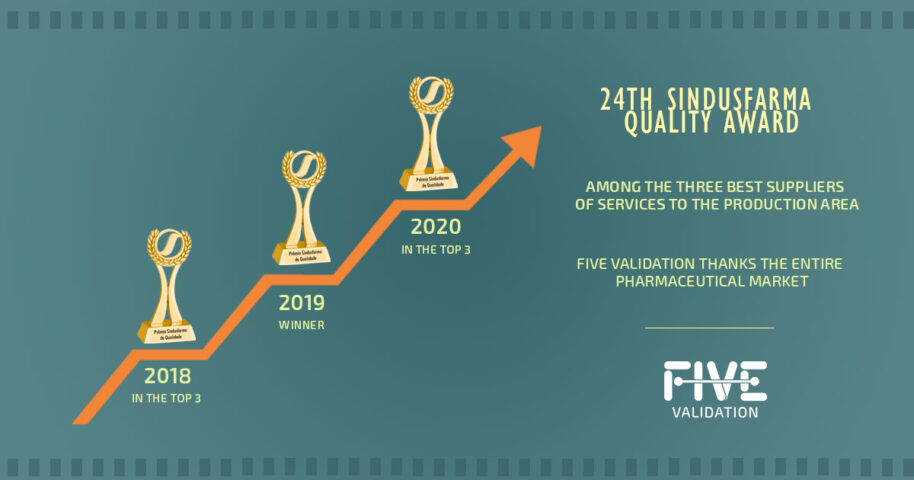
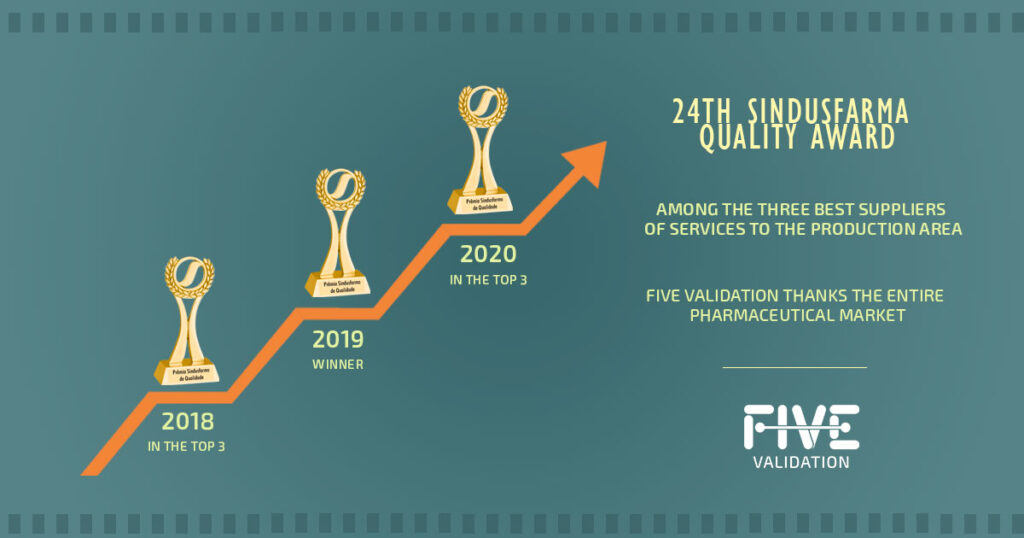
The First Participation in the SINDUSFARMA Awards 2018 Ceremony

In 2018, FIVE enrolled for the first time to participate in the 22nd SINDUSFARMA Quality Awards. After FIVE implemented its Quality System, a new category in the awards ceremony was announced. This award category was, and still is, an initiative of SINDUSFARMA (Brazil’s Pharmaceutical Industry Union) that evaluates suppliers and service providers in the pharmaceutical industry to recognize those with the best Quality System performance. FIVE received the most votes and ranked in the top three. FIVE's participation in the 22nd SINDUSFARMA Quality Awards Ceremony was significant because FIVE knew that there were some improvement points that could be applied. The auditors of SINDUSFARMA performed an audit which served as the first guide for the improvement of quality systems in this area. All the auditors’ recommendations of improvements and identification of non-conformities were evaluated and worked on. This was one of the highlighted, positive points raised in the audit carried out in the following year by SINDUSFARMA.
These improvement points included: a lack of creation/management of indicators; a lack of evidence of continuous improvement; and a not so effective management system of deviation records.

Digitalization of the Quality System
Considering all the points raised in the audit, FIVE identified that a paper-based management of its Quality System could not allow an effective management and integration of all processes.
What was more, it was demanding too much time from professionals to perform activities.
Therefore, FIVE looked for solutions in the market to meet this need, and because of this, a partnership with the company Forlogic was initiated.
FIVE began to use the Qualiex system, a totally paperless quality management system that integrates all management processes in a single tool.
Since 2018, FIVE has worked hard to improve its quality management system; implement Qualiex; and to share the good practice of quality culture in the company.
First Time to Win an Award in the SINDUSFARMA 2019 Ceremony
In 2019, FIVE enrolled again to participate in the 23rd edition of the SINDUSFARMA Quality Awards Ceremony.
In that year’s audit, FIVE's collaborators presented the Quality System to SINDUSFARMA auditors who evaluated the new quality management format. The main positive points raised during that year’s audit were: the participants’ preparation; FIVE's concern in working on the items highlighted in the previous year's audit; and the accuracy of its documentation. At the end of that year’s event, FIVE was voted the best service provider for the pharmaceutical industry of 2019 in the category of ‘Service Provider for the Production Area’. This was an achievement much celebrated by FIVE's collaborators, which really was a recognition for everybody’s work involved.
After being awarded in 2019, FIVE focused on continuing the progress of its Quality System, working to ensure that trainings continued and were constantly updated; new documents were developed; deadlines were met; incidents were dealt with; indicator targets were met; and suppliers were evaluated.

Winner in the SINDUSFARMA 2020 Awards Ceremony
In that year, FIVE enrolled to participate in the 24th edition of the SINDUSFARMA Quality Awards Ceremony. During the pandemic, there was some uncertainty about the event, the audits and in which format of the ceremony would take place. However, the SINDUSFARMA team managed to have the event with some changes and as a novelty for 2020, the execution of the audit was done for the first time by KPMG, a third party who was contracted by SINDUSFARMA. Contrary to previous years, the 2020 auditors were volunteers from SINDUSFARMA partner companies.
KPMG did the audit remotely by videoconference in September. As FIVE's system is completely paperless, its presentation and records were processed smoothly, which was noted by the auditors as one of FIVE’s strong points. The actual ceremony took place on October 6th in remote format and FIVE was once again awarded as the best provider of production services for the pharmaceutical industry of 2020.
Actually, this award was a recognition of all the employees’ work, since without their collective collaboration, the quality system would have not happened. This award demonstrates that we are on the right path in our pursuit of continuous improvement. FIVE aims to obtain ISO 9001:2015 and ISO 27001:2022 certifications to demonstrate FIVE's compliance and commitment to quality service delivery. The focus is now on maintaining our already positive points and implementing improvements wherever possible, which will result in the provision of an even better service to our clients and partners.

Article written by Luana Ribeiro (Quality and Validation Coordinator).



