ISPE GAMP® Good Practice Guide: Enabling Innovation – Critical Thinking, Agile, IT Service Management.

Using a Waterfall model is a very common practice for project delivery of paper-based validation, or validation using a text editor. However, this results in a great amount of extra effort and time. The average release and launch time for innovation in the Life Science industry can extend over months or years. Under the pressure, teams end up crunching testing phases to compensate for earlier phases that have been delayed. This can present a business risk.
The waterfall model here is not exactly the problem, there are several successful projects in this format. The issue is adaptation and flexibility.
ISPE's GAMP5™ guide for best practices describes the life cycle of GxP systems relevant to regulated Life Sciences companies and supports the use of incremental, evolutionary approaches, including Agile, for developing custom application products.
It is quite common for vendors to seek resale and increase the scope of their services, especially on the Cloud.
Much industry data now ends up being stored and managed by the vendor, who must prove their best practices by becoming a "co-regulator" (e.g., keeping the regulated company’s product, services, and/or patient data secure).
ISPE has released The ISPE GAMP® Good Practices Guide: Enabling Innovation – Critical Thinking, Agile, IT Service Management. It is the first guide in relation to the life sciences sector that addresses critical thinking, Agile, and IT service management.
Now professionals can adapt approaches, including the Agile model, and the needs of different systems, according to the complexity of each, while still complying with due compliance.
The Enabling Innovation guide seeks to apply ISPE GAMP®5 principles and current best practices in these areas to promote innovation and advancement. Part of the scope of this guide has also been included in the second edition of GAMP5.
Agile is a controlled process, and like the Waterfall approach, if executed poorly with a lack of control is unacceptable.
Some potential misconceptions around Agile and GxP, and the consequences that can arise are:
- 1) Vendors who use Agile for product development are then directed to deliver products that resemble the waterfall approach of a regulated company.
- 2) Statements from the Agile Manifesto that are taken literally. For example, "software development effort greater than documentation" does not mean “no documentation or records”.
- 3) References to documentation interpreted within a very narrow scope of traditional approved documents/specifications rather than also seeing them as records/information/artifacts within software tools.
Agile Framework in Validation
One tool that is already included in the Agile Validation Framework, which complies with FDA, EMA, and WHO is GO!FIVE®, an excellent option for agile projects:
- Create/define items per line matrix;
- Partial Release (per sprint);
- One validation plan and several partial reports.
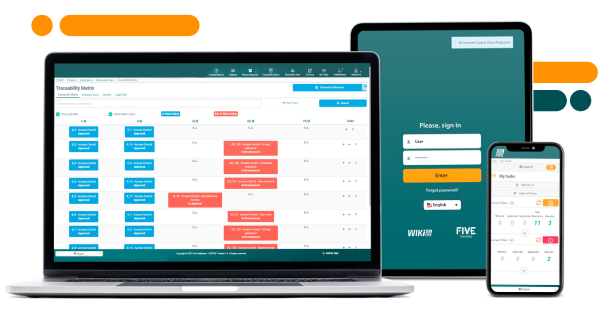
CLICK HERE to learn more about the system that brings Agile compliance and empowers teams.
Key Agile Concepts
Key Agile concepts are those that a regulated company, or a supplier to a regulated company, need to adopt:
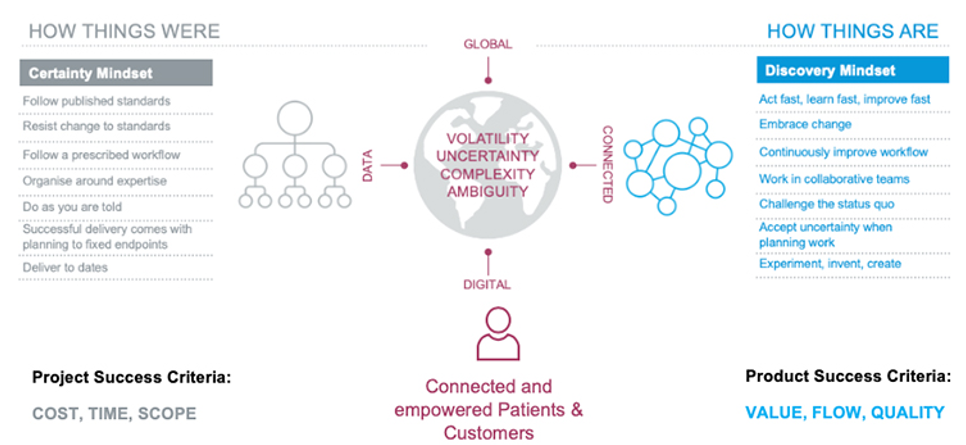
1.) The Discovery Mindset
To achieve this, we should look at long-term goals. But to get there, it should be through small effective changes, predicting and taking into consideration the near future.
The advances in technologies and the speed at which they have occurred, along with the uncertainties that they present, have forced organizations to rethink business models.
This applies to the process owner and the quality groups, where in a traditional approach, both tend to only be involved at a detailed level (e.g., approve all requirements of the URS, and/or review all test results).
2.) From requirement to product
Then, the procedure is to develop/configure, test, a release in repetitive cycles.
Although this is different from what regulated companies are usually used to, teams can develop applications in a controlled manner that conform to GxP requirements by applying the Agile framework.
In this case, a consistent and complete set of requirements are defined (e.g., epics and user stories) and are verified before a system is released, intended to be used in a GxP environment.
Agile versus waterfall
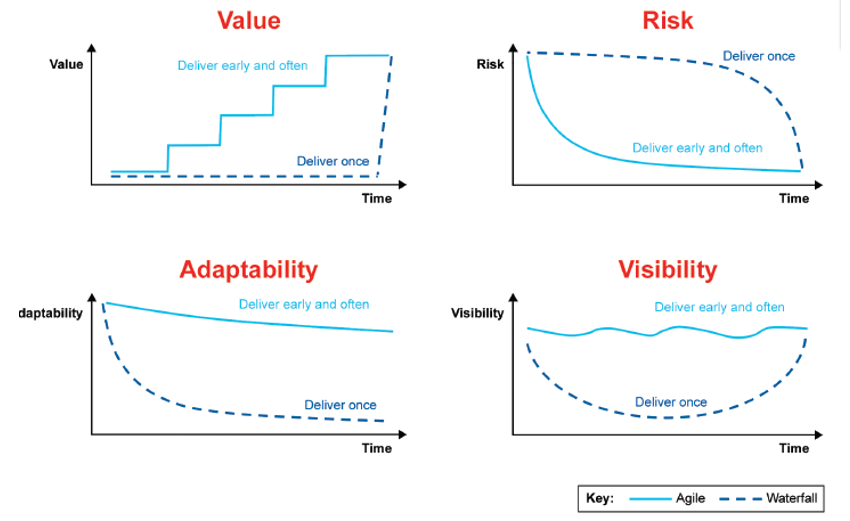
Early and recurring releases can help you deliver value sooner by getting feedback on the performance of those releases and thereby reducing the risks associated with delivery.
It helps to increase adaptability and responsiveness to changing needs and improves visibility for staff and customers regarding progress, as well as providing an early return on investment.
3.) Tools instead of documents
• Tools that allow the team to write functional and quality requirements efficiently, jumpstarting the agile process.
• Testing is an excellent example of where software tools can provide opportunities to improve delivery performance.
• A high volume of tests is executed more efficiently, with fewer human efforts.
• A tool that can provide automatic traceability without the need to create a separate matrix document manually.
• Traceability can be dynamically achieved and demonstrated solely by the tool, which, if required, can generate reports at key milestones (i.e., partial releases).
4.) DevOps and Continuous Integration
Continuous Integration (CI) and Continuous Deployment (CD), also known as “Continuous Delivery,” is the distinction between ‘continuous delivery’ and ‘continuous deployment.’ There is still human oversight/approval for the final deployment to the production environment, a critical and relevant “GxP Best Practices”.
I hope you enjoyed this article. If you want to know more about how to apply Agile in validation/qualification projects, please contact our experts at [email protected].
GAMP5® is a guide with intellectual rights reserved to the ISPE. Available for purchase at https://ispe.org/.
References used
- FDA 21 CFR Part 11
- GAMP5™ 1st and 2nd
- GAMPTM Good Practice Guide: Enabling Innovation, 2021
- https://ispe.org/pharmaceutical-engineering/ispeak/agile-software-development-gxp-regulated-environments-gamp



