Navigating the Regulatory Challenges of Medical Devices and Health Tech Startups: Embracing Agile and Digital Validation for Faster Time-to-Market

A startup's path to growth involves numerous obstacles and stages, particularly for those operating in regulated markets such as Medical Devices and Health Tech.
One of the biggest challenges they face is determining the optimal time to release their product, accounting for factors such as innovation, development, and regulation.
Without validation, the biopharmaceutical and medical device industries cannot register or produce their products.
Startups and scaleups must adhere to the same regulatory and validation standards as large corporations but with a significantly smaller workforce. Typically, startups do not have the expertise in-house or the resources to pay for consultants at the very early stages.
At the initial stage, most startups lack the knowledge and expertise in regulatory compliance, which can consume valuable resources such as time, money, and energy, risking the loss of their entire investment.
In a well-known study conducted by McKinsey & Co., it was revealed that a product delayed by six months before hitting the market experiences a significant loss of 33% in profits over a five-year period.
Alternatively, in a different scenario, when the same product is launched as planned but with a 50% budget overrun, the impact on profits is considerably less, with only a 4% reduction over the same five-year span.
To summarize:
Scenario 1:
Budget: Within budget
Time: Six months behind the scheduled date
Impact: 33% reduction in profits over five years
Scenario 2:
Budget: 50% over budget
Time: Launched on the scheduled date
Impact: 4% reduction in profits over five years
Surely it is possible to imagine the gain that is obtained when we reduce the time of availability of a product to market, without leaving aside quality and compliance within budget. This is why speed in validation activities is a competitive advantage.
One should not forget that in addition to the innovation period, there is the documentation effort, which can also take a considerable amount of time from the project.
This becomes even more dangerous considering that startups usually do not generate revenue yet. Therefore, such an impact can even mean the end of the company.
Taking this into account, it is highly recommended that regulated startups apply a critical thinking culture aligned with ICH Q9 Quality Risk Management principles, which basically supports informed decision-making and good judgment on the extent and depth of activities (e.g.: level of documentation formality).
As mentioned before, without validation it is not possible to make biopharmaceuticals and medical devices available on the market. So, streamlining the process can be key to making a difference in a startup.
Besides, it is common knowledge that market pioneers enjoy some advantages in terms of market share, revenue, and economic growth.
Startups can also gain knowledge from consultants, learn as they go, and build on employees; however, considering as soon as possible the digital approach allows the resources to focus on higher-value tasks: more time to innovate and develop new processes, and the entire team will have engaged quality, since data is easily accessible, and the requirements are clear and ready-made.
What is GAMP5®, second edition and CSA?
Life science companies, particularly those involved in biopharmaceuticals and medical devices, operate in a highly regulated environment where it is crucial to demonstrate their products' robustness and development processes.
Each startup in these industries must maintain procedures for product design control to ensure that specified requirements for the project are obeyed. Validation plays a vital role in this process by generating evidence that the product meets the user’s needs and indication of use. Design validation shall include software validation, when appropriate.
During the early phase, project validation holds significant importance. However, as the company advances and approaches the stage of obtaining the Good Manufacturing Practices (GMP) certificate, additional validations may become necessary. These validations might encompass spreadsheets, systems, and equipment, if applicable, that impact product quality, data integrity, or the safety of patients and/or consumers.
To guide the Life Science community in developing robust validations, the International Society of Pharmaceutical Engineering, ISPE® created the GAMP® Guide (Good Automated Manufacturing Practice, now in the second edition).
The GAMP5® Guide encourages using software tools to support Agile practices that can improve the traditional documentation approach, which can introduce non-compliance risks due to the difficulty in tracing paper documentation.
In addition, the FDA's Computer Software Assurance (CSA) guide, which was released in draft form in September 2022, focuses on assuring the quality of software used in the manufacturing process and quality assurance of medical devices.
Despite differences in their guidelines, the GAMP5® and CSA share an interesting synergy regarding the scope of tests. Both guides suggest or recommend similar types of guided and unguided testing, indicating a common understanding of the importance of ensuring the safety and efficacy of Life Science products.
To sum it up, both guides advocate critical thinking and emphasize the utilization of software tools. While the CSA does not place its primary focus on assessing patient risk, GAMP5® stands out as a robust methodology that enjoys universal acceptance among regulatory agencies worldwide. Consequently, it can and should be regarded as an excellent guideline for validating finished product software.
Waterfall vs Agile
In the Life Science industry, it is common to use the Waterfall model, in cascade, for project delivery, often because of the workload involved in paper or digital manual validation (based on a text editor).
With Waterfall software development, there is a linear flow of defining/collecting all requirements before transforming them into a complete set of functional and design specifications that are coded/configured before testing commences.
Nevertheless, this approach can result in substantial delays, extending the time required for the release and launch of innovations to several quarters or even years. A standard R&D medical device project already spans multiple years; however, without a preconceived validation strategy, this timeline can be further extended.
In addition to validation/qualification being a requirement for the registration and commercialization of products, a robust set of documents can get your approval faster and without adjustments by making the regulatory bodies understand better the innovation proposed by your product.
In another study released by McKinsey & Company, an intelligent quality system can have a tangible impact on profits and accelerate time-to-market by more than 30% and increase the supply chain production capacity by 20-30%.
Smart quality combines advanced technologies, standardization, process, and flexible ways of working that are totally aligned with the Agile Framework.
With Agile software development, requirements are collected and then moved into development/configuration, testing, and release in iterative cycles.
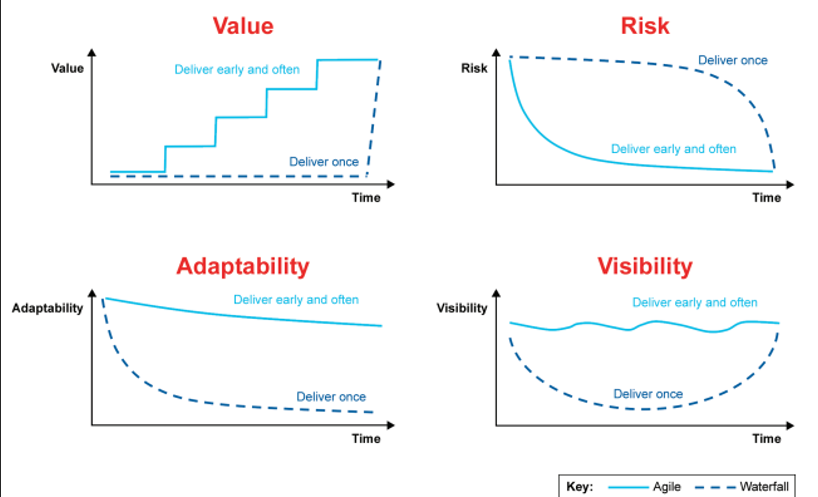
It is not linear approaches that are the issue, but not undergoing constant change. To achieve this mindset, long-term goals need to be considered, but progress toward them should be through small effective changes.
Click here to learn more about Agile concepts and how they can help streamline the delivery of projects in the Life Science industry.
Navigating the Regulatory Landscape: A Strategic Approach for Startups and Scaleups
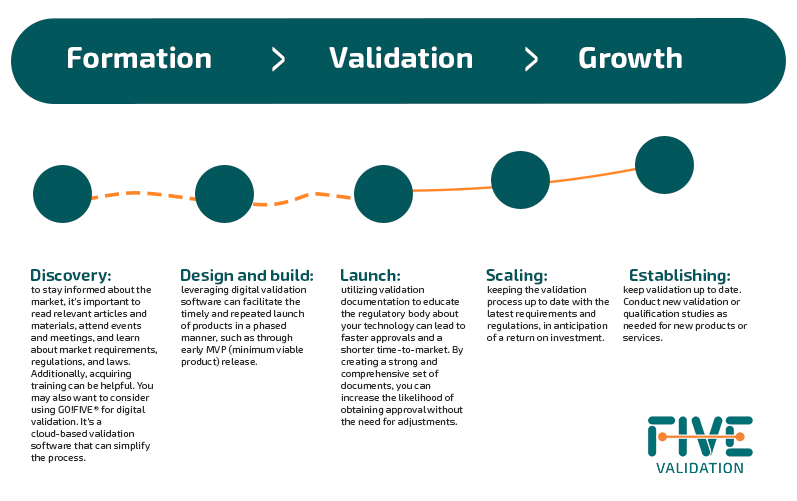
While transitioning from one stage to another (discovery, design and build, launch, scaling, and establishing) can be challenging, various approaches can be pursued to attain this objective.
Having trustful partners who comprehend the market is crucial for startups to save time and money, irrespective of their stage .
Moreover, adhering to and knowing GxP requirements (impact on good practices) set by regulatory bodies such as FDA, EMA, and WHO since the beginning will ensure that the solution is being built in a controlled manner.
Considering this, we have created the GO Startup Program, which provides a comprehensive package offering software, training, support, and consulting hours to help you comply with FDA, EMA, and WHO validation requirements, 5 times faster.
As a result, entering a highly regulated market should be viewed as a strategy rather than a disconnected set of tactics.
The Importance of Digital Validation in the Pre-Seed and Seed Phases of Investment for Startups
It is recommended to engage in intensive reading of articles and materials, as well as participate in events and meetings that cover the market's requirements during the pre-seed investment phase.
It is crucial to familiarize yourself with the regulations of the countries where you plan to market your product.
At this stage, obtaining training can provide valuable knowledge and skills at a relatively low cost.
In this phase, it is crucial to identify market risks and opportunities, as well as the products that can address them, as exemplified below:
Performing validation on paper entails several risks, such as:
- Lack of validation content and references
- Possible rejection of documentation by regulatory agents due to lack of robustness
- Longer time-to-market resulting in lost revenue.
- Limited flexibility in releases and changes due to restricted, prescriptive document templates.
If you opt for digital validation that meets the Agile framework with GO!FIVE®, you can take advantage of various opportunities, including:
- Delivering value sooner with early and recurring releases, leading to an early MVP.
- Obtaining faster approvals with a robust set of documents, resulting in time-to-market advantages.
- Improving visibility for both staff and clients.
- Explaining the technology involved.
- Achieving a return on investment in advance.
It's not uncommon, particularly when dealing with truly innovative concepts, to encounter unforeseen or unknown elements that necessitate adjustments to the project.
That's why boosting adaptability and responsiveness to changing needs is highly strategic from the seed phase onward.
When implementing digital validation in the design and build phase, this adaptability becomes a competitive advantage, since it is widely recognized that early adopters in the market can benefit from a bigger market share, revenue, and economic growth.
Hospitals holding best practice certifications may demand the submission of product validation documentation for medical devices with embedded software such as X-ray, ultrasound, CT, or MRI machines. Maintaining thorough and current documentation can give a competitive edge.
Keeping that in mind, GO!FIVE® software enables performing partial releases per sprint and provides the flexibility to make dynamic changes to the content of these releases if needed during the project.
Additionally, the function for releasing and versioning items separately facilitates maintaining their validated state while treating new and existing items differently.
Revisiting validation is a recurring process that must be done with each new software version and process change. Nevertheless, the system consolidates the changes and versions of these items.
By doing so, the startup can automatically maintain the traceability of each process stage, eliminating the confusion caused by outdated versions and the need to highlight each new change, thus saving more time to dedicate to other activities.
Using GO!FIVE®, you can maintain a comprehensive log of all changes made while still having a clear overview of what is currently in effect by generating these deliverables.
This approach is particularly beneficial for those seeking to maintain organized and up-to-date project documentation.
Do you want to start your digital validations?
Utilizing our database, which has over 15 years of experience in the field, can help save time and money that would otherwise be spent building and maintaining validation documents.
In addition to the system's extensive knowledge base, one can seek assistance from our experts to quickly initiate and develop their validation/qualification projects.
Do you want to talk with our specialists? Click here to schedule a meeting.
About the author: Lilian Ribeiro
She has nearly four years of technical and business experience in the food industry, specializing in corporate quality/quality control. Additionally, she has three years of experience in the health/pharmaceutical sectors.
As a paperless validation enthusiast, she is passionate about bringing efficiency and innovation to Life Science companies.
Her expertise lies in validation and qualification projects, including VLMS, ERPs, EQMS, automation (PW), and IT Infrastructure Qualification.
About the reviewer: Silvia Martins
She is an electrical engineer with 20 years of experience serving Biopharmaceuticals and Medical Devices companies. She received training in GAMP5 and FDA 21 CFR Part11 in England, SAP® validation in Germany, and Data Integrity and Data Governance in Denmark.
As CEO and co-founder of FIVE Validation, a company dedicated to streamlining compliance processes, I am committed to making compliance faster and simpler for our clients.
About the Reviewer: Rodrigo Olmedo, from uGlobally
Rodrigo Olmedo is a Brazilian entrepreneur with a marketing and international business background. He co-founded two companies, including uGlobally, an organization focused on helping tech companies expand globally. Rodrigo has worked with over 600 influential ecosystem players and startups from more than 40 nationalities, supporting governments, corporates, and tech companies in various aspects of international expansion. Additionally, Rodrigo serves as a business advisor, speaker at events, facilitator of European projects, and mentor for accelerator programs worldwide.
References:
GAMP5® second edition 2022
ISPE GAMP® Good Practice Guide: Enabling Innovation – Critical Thinking, Agile, IT Service Management.
https://www.forbes.com/sites/steveblank/2018/10/08/what-startups-need-to-know-about-regulated-markets/?sh=14b938fc70cd
https://www.sipa.columbia.edu/news/experts-discuss-startups-and-regulated-markets
https://inatel.br/blog/empreendedorismo/252-quero-iniciar-uma-startup-por-que-devo-fazer-a-ideacao
https://hackmed.com.br/blog/fases-de-uma-startup/
https://www.mckinsey.com/industries/life-sciences/our-insights/smart-quality-reimagining-the-way-quality-works
SAP® is a registered trademark of SAP® in Germany and other countries. All rights reserved. For more details, access https://www.sap.com/corporate/en/legal/trademark.html
GAMP5® is a guide that has its intellectual rights reserved by ISPE™. Available for purchase at https://ispe.org/.



