What is paperless validation?


Paperless validation is an electronic process of ensuring that equipment, software, spreadsheet, utilities, cleaning, and processes, including the series of tests in Life Science companies are compliant to the regulatory bodies such as FDA, EMA, and WHO.
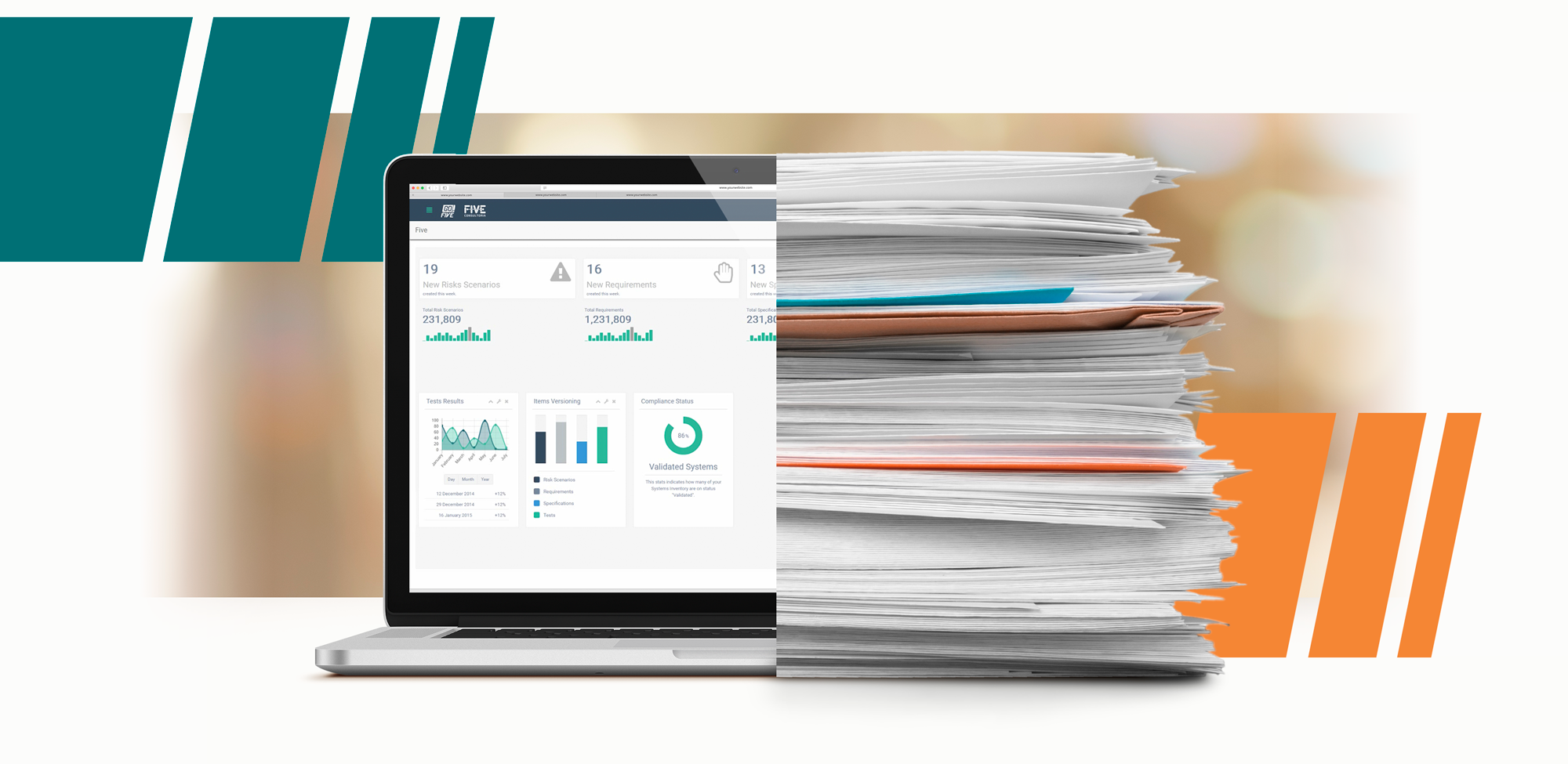
All steps of the validation process are registered in digital documents, or items, rather than on paper, as in the classic computer system validation FDA format.
Paperless validation offers numerous benefits for businesses, professionals, and the environment, far beyond mere paper savings.
10 reasons to consider paperless validation software.
1.) More compliance: decrease regulatory risks to businesses and data integrity.
2.) Faster time to market: with no validation, biopharma and medical device industries cannot register or produce their products.
3.) More efficient work: ‘right the first time;’ decrease the time to compliance, make projects agile, and possess ‘a knowledge database’.
4.) Decrease validation costs: faster work, avoid paper work, no printers, no physical space to store documents, no documentation scanning.
5.) Remote work: healthier staff and quality of life, online management, connect teams between several countries.
6.) Easier validation status maintenance: decrease the time to keep validation status with constant update and periodic inspections.
7.) Easier audits: immediate availability of data.
8.) Standard documents: maintaining good documentation practices according to GMP guidelines, GAMP5, for example.
9.) Easier management: immediate availability of data (online management)
10.) Sustainable: no use of paper, no printers, no cartridge disposal
Traceability Matrix and Test Application

Traceability matrix is a document that links GMP requirements with their tests over the validation lifecycle. This deliverable ensures that all requirements defined for a system, software, and process validation are linked with their respective risk scenarios and tests.
In a traditional way, based on paper, the Analyst needs to link all requirements with their test protocol items manually.
Furthermore, test application is extremely fast compared to the traditional paper-based method, as the GO!FIVE® software automatically organizes all the evidence for each test.
By using a paperless validation software like GO!FIVE®, the traceability matrix is automatically generated, reducing time, and avoiding mistakes.
Data Integrity
Data Integrity in a validation project refers to ensure that the data inserted in those documents, such as 21 CFR Part11 test scripts are attributable, legible, contemporaneous, original, and accurate. In case of some changes in data, the Validation Analyst should review all paper documents manually.
If one conducts their project using a paperless validation software, the chances of a break of GxP compliance are smaller. The data inserted on the platform has integrity when using GO!FIVE®.

With ESG (Environmental, Social, and Governance) in validation, the company demonstrates:
The company shows the care with your professionals.

With paperless validation software, the validation team can perform their tasks either remotely or on-site, bringing more flexibility to the professionals' planning. This reduces the need for commuting from home to the company, directly impacting the quality of life for employees who save travel time and the use of vehicles that emit CO2.
Respect for the environment
With digital validation, the process is sustainable, as there is zero paper consumption. In contrast, paper-based validation, especially for widely used systems like ERP (Enterprise Resource Planning), can require around 2,500 sheets of paper for a single validation.
This means paperless validation conserves valuable trees and all the natural resources of water and electricity used in paper production.
Additionally, it eliminates the need for printers and their toner cartridges, scanners for document scanning, folders for organization, and physical space for storage.
Moreover, and no less important, when we avoid commuting and the use of vehicles, we reduce CO2 emissions.
Time spent to validate
With GO!FIVE®, it is possible to complete the validation process from start to finish within a single software, with the advantage of increasing the speed of validation projects through paperless methods. Better than an EDMS (Electronic Document Management System), which is commonly used for validation projects. EDMS still needs paper documents management during test run phases.
The possibility of conducting a digital validation project within a single software also translates into financial benefits for Life Sciences companies, as using paperless validation software reduces the team's efforts to complete the project, saving 80% of its validation costs!
Now, it becomes clearer that paper-based validation comes with high costs! With GO!FIVE®, the paperless validation software, you save time (6x faster) and financial resources!

Agile compliance that empowers your team!
GO!FIVE® is a balanced software platform. It reduces repetitive and mechanical activities, leaving the thinking activities like Risk Assessment and testing strategies for the validation specialist to decide.
It has a library, which is a compilation of all the knowledge acquired by FIVE in more than 1000 validation projects, anonymized and built into the database with pre-formatted information about each topic.
However, your validation specialist has the authority to determine whether these items conform to your process and project, while benefiting from an improved process by leveraging the information stored in the database, which is constructed and continually enhanced by experts.
The validation professional will start their work from a knowledge base, meaning they won't begin from scratch with each new project, saving a significant amount of time and enabling validations that are at least 6 times faster.




