Webinar – Remote Validation

This webinar event was broadcast on Oct 28th 2020
GAMP5® is a guide that has its intellectual rights reserved by ISPE™. Available for purchase at ispe.org
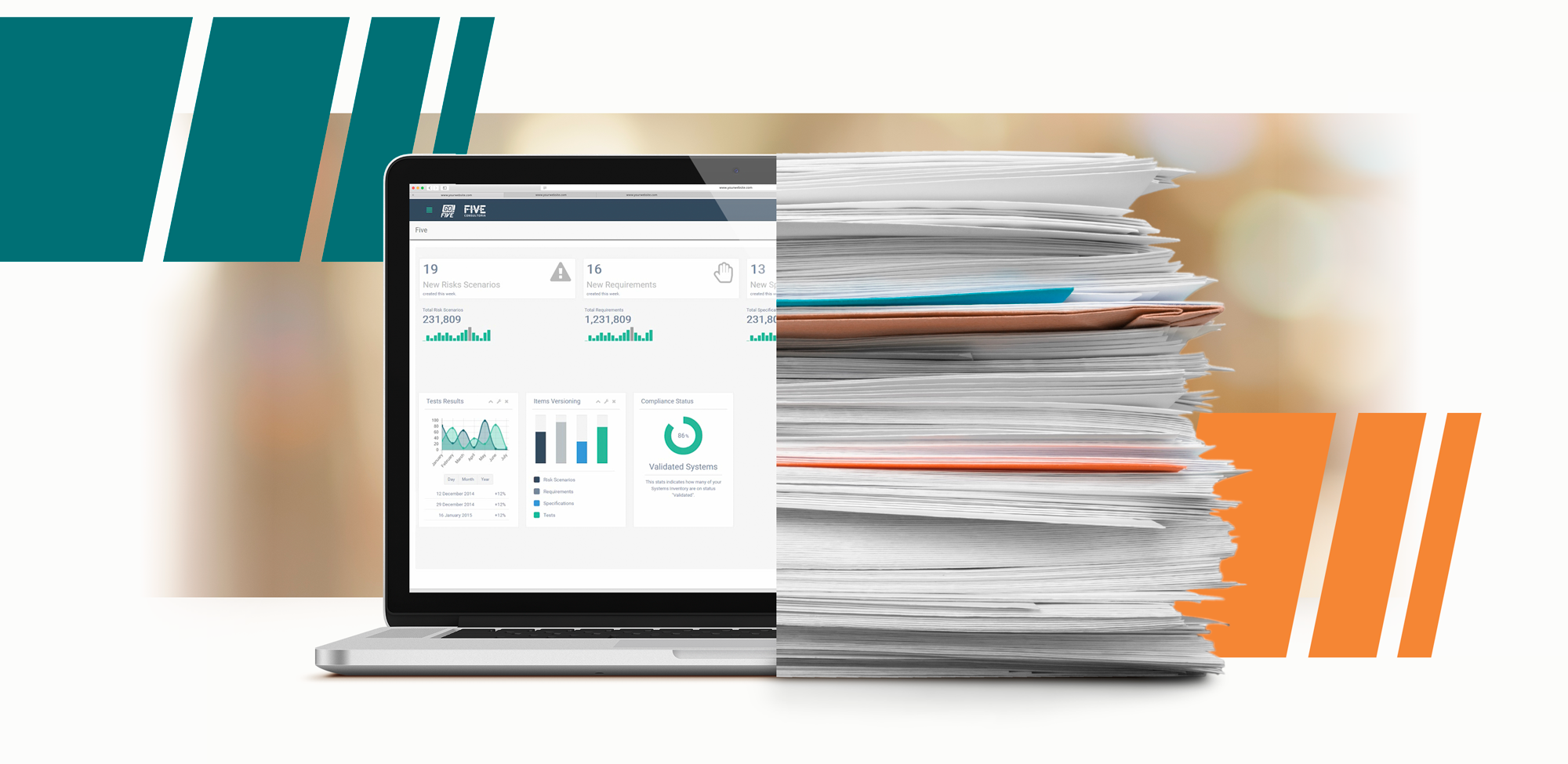
Qualification and Validation: Regulatory agencies such as FDA, EMA and WHO, require that the pharmaceutical, medical device and supply chain industries prepare documents that prove the proper functioning of processes, systems, and equipment.
Traditionally, qualification and validation are documented on paper, then filed in binders or folders. Some companies use a set of electronic systems to cover all validation lifecycle documents.







